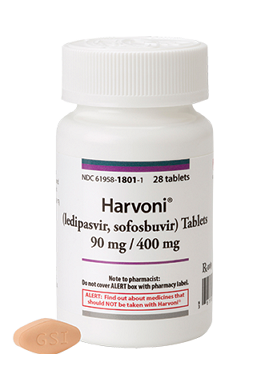
Harvoni Tablets Gilead Sciences, Inc.
$50.00
Here are Harvoni (Ledipasvir/sofosbuvir) costs in :
– the US ($94,500)
– Canada ($80,000)
– India ($900)
– the UK (£39,000)
– Germany (48,000€)
– Egypt ($1200)
This medicine is a mix of ledipasvir and sofosbuvir and is utilized to treat incessant (durable) hepatitis C, a viral contamination of the liver. It might once in a while be utilized with another antiviral medicine (ribavirin). These medications work by lessening the measure of hepatitis C infection in your body, which enables your insusceptible framework to battle the contamination and may enable your liver to recuperate. Endless hepatitis C contamination can cause genuine liver issues, for example, scarring (cirrhosis), or liver tumor.
It is not known whether this treatment can keep you from passing the infection to others. Try not to share needles, and practice “more secure sex” (counting the utilization of latex condoms) to bring down the danger of passing the infection to others.
Step by step instructions to utilize Harvoni
Read the Patient Information Leaflet if accessible from your drug specialist before you begin taking ledipasvir/sofosbuvir and each time you get a refill. On the off chance that you have any inquiries, ask your specialist or drug specialist.
Take this pharmaceutical by mouth with or without nourishment as coordinated by your specialist, ordinarily once every day.
This pharmaceutical works best when the measure of medication in your body is kept at a steady level. In this way, take this medication at uniformly divided interims. To enable you to recollect, take it in the meantime every day.
Tell your specialist on the off chance that you upchuck inside 5 hours in the wake of taking this prescription. You may need to take another dosage.
Keep on taking ledipasvir/sofosbuvir for the full timeframe endorsed, regardless of the possibility that manifestations vanish a little while later. Ceasing the medicine too soon may bring about an arrival of the disease.
Acid neutralizers bring down the retention of ledipasvir. On the off chance that you are taking an acid neutralizer, take it no less than 4 hours earlier or 4 hours after this prescription.
Other corrosive bringing down meds for acid reflux, indigestion, or ulcers, (for example, medicine or over-the-counter meds including famotidine, omeprazole) may keep ledipasvir from working. Ask your specialist or drug specialist how to utilize these medicines securely.
Doses & Administration
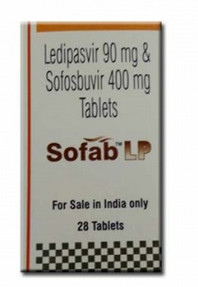
Each tablet contains 90 mg ledipasvir and 400 mg sofosbuvir. Tablets include the following inactive ingredients: colloidal silicon dioxide, copovidone, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. These tablets are film-coated with a coating material containing the following inactive ingredients: FD&C yellow #6/sunset yellow FCF aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
It is indicated with or without ribavirin for the treatment of patients with chronic hepatitis C virus (HCV) genotype 1, 4, 5, or 6 infection.
Harvoni Side Effects:
If Harvoni is administered with ribavirin, refer to the prescribing information for ribavirin for a description of ribavirin-associated adverse reactions. The safety assessment was based on pooled data from three randomized, open-label Phase 3 clinical trials (ION-3, ION-1 and ION-2) of subjects with genotype 1 HCV with compensated liver disease (with and without cirrhosis) including 215, 539, and 326 subjects who received once daily by mouth for 8, 12 and 24 weeks, respectively.
The proportion of subjects who permanently discontinued treatment due to adverse events was 0%, less than 1%, and 1% for subjects receiving these tablets for 8, 12, and 24 weeks, respectively.
The most common adverse reactions (at least 10%) were fatigue and headache in subjects treated with 8, 12, or 24 weeks of Harvoni.
Potential For Drug Interaction
As Harvoni contains ledipasvir and sofosbuvir, any interactions that have been identified with these agents individually may occur with tablets.
After oral administration of tablets, sofosbuvir is rapidly absorbed and subject to extensive first-pass hepatic extraction. In clinical pharmacology studies, both sofosbuvir and the inactive metabolite GS-331007 were monitored for purposes of pharmacokinetic analyses. Ledipasvir is an inhibitor of the drug transporters P-gp and breast cancer resistance protein (BCRP) and may increase intestinal absorption of coadministered substrates for these transporters.
Ledipasvir and sofosbuvir are substrates of drug transporters P-gp and BCRP while GS-331007 is not. P-gp inducers (e.g., rifampin or St. John’s wort) may decrease ledipasvir and sofosbuvir plasma concentrations, leading to reduced therapeutic effect of Harvoni, and the use with P-gp inducers is not recommended with Harvoni.
- Free delivery over 00
- 30 Days Return Period

Customer Reviews
There are no reviews yet.